There are three main ways of forming chemical bonds between atoms. This year, we only had to learn the first two chemical bondings.
- Ionic Bonding, which results when electrons are transferred from one atom to another forming positive and negative ions.
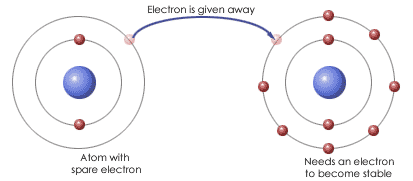
-Covalent Bonding, which resulta when atoms re joined together by sharing electrons, forming molecules.

-Metallic bonding, which is found only in metals.

Valency Electrons:
The electrons in the other shell are used to form bonds between atoms. These electrons are called valency electrons. The number of electrons are called valency electrons. The number of electrons an atom uses to form bonds is called its valency. Hydrogen uses its only electron to form bonds, so it has a valency of 1. Oxygen uses two electrons to form bonds so it has a valency of 2. Carbon uses all of it's four outer electrons to frm bonds, so it has a valency of 4.
No comments:
Post a Comment